Table 1 gives some properties of the elements in period 3 of the periodic table.
| Element | Atomic Number | Atomic Radius (nm) |
|---|---|---|
| Na | 11 | 0.186 |
| Mg | 12 | 0.160 |
| Al | 13 | 0.143 |
| Si | 14 | 0.117 |
| P | 15 | 0.110 |
| S | 16 | 0.104 |
| Cl | 17 | 0.099 |
| Ar | 18 | 0.097 |
(a) Give the formula and name of the compound formed by the reaction between Al and S.
Formula: Al2S3
Name: Aluminium Sulphide
(b) Explain the variations in the atomic radius of the elements across the period.
The atomic radius decreases across the period. This is due to the increase in the atomic number while the number of energy levels remain the same across the period.
(c) Select the element with the highest ionization energy. Give a reason.
Argon, Ar. The ionization energy of elements in the period 3 increases across the period.
Argon has a stable electron configuration.
(d) Write the electron arrangement of phosphorus in PCl5.
2, 8, 5
(e) Select an element that forms an ion with the smallest ionic radius. Give a reason.
Aluminium (Al)
Aluminium loses three electrons to form an ion thus has a stronger effective nuclear charge that attract the outermost energy level closer to the nuclear making its ionic radius smaller compared to other elements in period 3
Aluminium/Al
Reason: It loses the highest number of electrons /3 electrons hence has the strongest nuclear pull for the remaining electrons
(i) Table 2 gives the melting points (°C) of some of the elements.
| Element | Melting Point (°C) |
|---|---|
| Na | 98 |
| Mg | 650 |
| Cl | -101 |
| Ar | -189 |
Explain, in terms of structure and bonding, the differences in the melting points of:
(i) Na and Mg:
Magnesium/Mg has more (2 delocalised electrons) than sodium/Na (1 delocalised electron) hence has a stronger metallic bond (that require more energy to break) than Na
(ii) Cl and Ar:
Chlorine/Cl2 is diatomic (has larger molecules) while Argon/Ar is monoatomic; hence Cl2 has more Van der Waal's forces therefore more energy is required to break them
2 (a) Complete the following equation:
CaC2(s) + H2O(l) → Ca(OH)2(aq) + C2H2(g)
Pent-1-yne, \(CH≡C−CH_{2}−CH_{2}−CH_{3}\), reacts with bromine to form compounds B and C as shown in Figure 1.
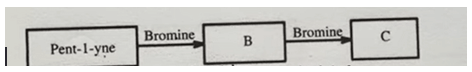
Compound B
\(CHBr=CBr−CH_{2}−CH_{2}−CH_{3}\)
Compound C
\(CH_{2}Br−CBr_{2}−CH_{2}−CH_{2}−CH_{3}\)Study the flowchart in figure 2 and answer the questions that follow
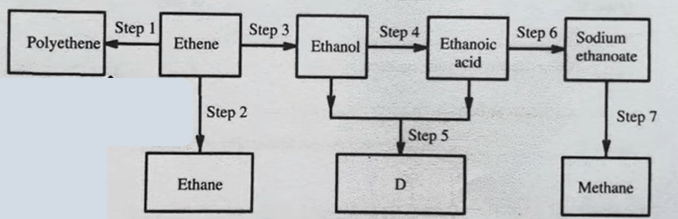
Give the reagents and conditions used in:
I. Step 2:
Reagent: Hydrogen
Conditions: Nickel Catalyst / Palladium Catalyst
Temperature: \(150^{o}C -300^{o}C\)
Pressure (1–100 atm)
Step 7:
Reagent: Soda lime
Condition: Heating
(ii) Write an equation for the reaction that takes place in:
Step 1:
CH2=CH2 + CH2=CH2 → [-CH2-CH2-CH2-CH2-]
Step 3:
C2H4 (g) + H2O (g) → C2H5OH (l)
Alternative:
CH2=CH2 + H2O (l) → CH3-CH2OH
(iii) Name the type of reaction that takes place in Step 3:
Reaction Type: Hydration reaction (addition of water).
(a) Explain how an increase in temperature affects the rate of a chemical reaction. (2 marks)
An increase in temperature increases the rate of the reaction.
An increase in temperature increases the kinetic energy of the particles leading to more effective collisions.
(b) Consider the following gaseous reaction:
\( 2NO(g) + H_2(g) \rightarrow N_2O(g) + H_2O(g) \)
Explain how an increase in pressure affects the rate of this reaction. (2 marks)
...
(c) At high temperatures, \( NO \) and \( CO \) gases react as shown in the following equation:
\( NO(g) + CO(g) \rightarrow NO_2(g) + CO_2(g) \)
The reaction was monitored by measuring the changes in the concentration of \( NO \) with time. Table 3 shows the data obtained:
| Time/seconds | Concentration of \( NO \times 10^7 \) / moles per litre |
|---|---|
| 0 | 0 |
| 50 | 16 |
| 100 | 22 |
| 150 | 26 |
| 200 | 29 |
| 250 | 31 |
| 300 | 32 |
5(a) Use the standard electrode potentials in Table 5 to answer this question.
| Number | Electrode Reaction | E0, V |
|---|---|---|
| I | 2H+(aq) + 2e- → H2(g) | 0.00 |
| II | Zn2+(aq) + 2e- → Zn(s) | -0.76 |
| III | Sn2+(aq) + 2e- → Sn(s) | -0.14 |
| IV | Cu2+(aq) + 2e- → Cu(s) | +0.34 |
| V | Fe2+(aq) + 2e- → Fe(s) | -0.44 |
| VI | Pb2+(aq) + 2e- → Pb(s) | -0.13 |
| VII | Cu+(aq) + e- → Cu(s) | +0.52 |
| VIII | Ag+(aq) + e- → Ag(s) | +0.80 |
(i) Select two electrodes which when connected gives the cell with the lowest e.m.f.
III and VI
(ii) Arrange the metals Ag, Fe, and Sn and in order of their reactivity with dilute hydrochloric acid, starting with the most reactive. Give a reason.
Fe, Sn, and Ag. Iron is the most reducing agent, followed by tin, and lastly silver.
(iii) An electrochemical cell is made up of electrode numbers IV and VII.
I. Calculate the e.m.f of the cell.
E°(cell) = E°(reduction) - E°(oxidation)
= +0.52 - (+0.34)
= +0.18 V
II. Write an equation for the cell reaction.
Cu(s) + 2Cu+(aq) → Cu2+(aq) + 2Cu(s)
III. Draw a labelled diagram of an electrochemical cell that is used to measure the standard electrode potential for tin (Sn), electrode number III
.png)
(b) The products of electrolysis of sodium chloride depend on the conditions used. Give the products obtained under each set of conditions in Table 6.
| Conditions | Product at: Anode | Product at: Cathode |
|---|---|---|
| Dilute aqueous sodium chloride | 2Cl-(aq) → Cl2(g) + 2e- | 2H+(aq) + 2e- → H2(g) |
| Concentrated aqueous sodium chloride | 2Cl-(aq) → Cl2(g) + 2e- | 2Cl-(aq) + 2e- → Cl2(g) |
(c) Aqueous chromium(III) sulphate was electrolysed using inert electrodes. The equation for the reaction is:
Cr3+(aq) + 3e- → Cr(s)
Calculate the time in seconds required to deposit 2.6 g chromium using a current of 5.5 amperes. (1 Faraday = 96,500 Coulombs; Cr = 52.0)
Q = (M x n x F) / RAM
Q = (2.6 x 3 x 96,500) / 52.0
Q = 14,475 C
Time (t) = Q / I
t = 14,475 C / 5.5 A
t = 2632 seconds
6.(a) (i) State Charles' law of gases. (1 mark)
Charles' Law states that the volume of a given mass of gas is directly proportional to its absolute temperature when pressure is held constant.
(ii) Table 7 shows the data obtained in an experiment using 0.012 moles of neon gas.
Table 7
| Temperature/ K | Volume/dm³ | Pressure/atm |
|---|---|---|
| 250 | 0.005 | 50 |
| 300 | 0.006 | 50 |
Show that the data is consistent with Charles' law. (2 marks)
According to Charles' Law: The volume of a fixed mass of a gas is directly proportional to its absolute temperature at a constant pressure.
\( V \propto T \) at constant \( P \) OR \( V = kT \)
\( \frac{V_1}{T_1} = \frac{0.005}{250} = 2 \times 10^{-5} \)
\( \frac{V_2}{T_2} = \frac{0.006}{300} = 2 \times 10^{-5} \)
\( \Rightarrow \frac{V_1}{T_1} = \frac{V_2}{T_2} \) is a constant
Since the ratio of Volume to Temperature is constant, the data is consistent with Charles' Law.
(b) (i) State Graham's law of diffusion of gases. (1 mark)
The rate of diffusion of a gas is inversely proportional to the square root of its density at constant temperature and pressure.
(ii) Given that 1 mole of a gas occupies a volume of 24.0 dm³ at 298 K, calculate the density in grams per litre of:
I. oxygen gas (O = 16)
RMM of O2 = 16 x 2 = 32
NOTE: Density = \( \frac{Mass}{Volume} \)
-Density of O2 = \( \frac{32}{24} \)
= 1.333 g/L
II. hydrogen gas (H = 1.0)
RMM of H2 = 1 x 2 = 2
Density of O2 = \( \frac{2}{24} \) ✔️½ = 0.0833 g/L ✔️½
(iii) Determine the rate of diffusion of hydrogen gas compared to that of oxygen gas at 298 K.
\( \frac{Rate(H_2)}{Rate(O_2)} = \sqrt{\frac{Density~O_2}{Density~H_2}} \)
\( = \sqrt{\frac{1.333}{0.0833}} \)
= 4
∴ Rate H2 = 4 x Rate of O2
Hydrogen diffuses 4 times faster than oxygen gas.
6(c) Ammonia gas was prepared in the laboratory by warming a mixture of solid ammonium chloride and solid calcium hydroxide. The equation for the reaction is:
\( 2NH_4Cl(s) + Ca(OH)_2(s) \longrightarrow CaCl_2(s) + 2H_2O(l) + 2NH_3(g) \)
The gas was dried and then collected. If the volume of ammonia collected was 1340 cm³ measured at 312 K and 1 atmosphere pressure: (N = 14.0; Cl = 35.5; H = 1.0; Volume of one mole of gas at 298 K = 24 dm³)
(i) Calculate the volume that ammonia gas will occupy at 298 K and 1 atmospheric pressure.
\( \frac{P_1V_1}{T_1} = \frac{P_2V_2}{T_2} \) or \( \frac{V_1}{T_1} = \frac{V_2}{T_2} \) since P is constant
\( V_2 = \frac{298 \times 1340}{312} \)
= 1279.87 cm³
6 (c) (ii) Determine the mass of ammonium chloide that reacted
\( 2NH_4Cl(s) + Ca(OH)_2(s) \longrightarrow CaCl_2(s) + 2H_2O(l) + 2NH_3(g) \) 1279.87 cm³
Moles of NH3 = \( \frac{1279.87}{24000} \)
= 0.0533
Mole ratio NH4Cl : NH3 = 1 : 1
Moles of NH4Cl = 0.0533
RFM of NH4Cl = 14 + 4 + 35.5
= 53.5
Mass of NH4Cl = 0.0533 x 53.5
= 2.85 g
(b) Sodium carbonate is manufactured through a series of reactions involving sodium chloride, ammonia and carbon(IV) oxide.
(i) Ammonia is obtained by reacting hydrogen and nitrogen in the Haber process. State how the other two materials are obtained:
I. Sodium chloride;
Evaporation of sea water or
Mined as rock salt
II. Carbon(IV) oxide.
Heating(burning) limestone and coke or
Thermal decomposition of NaHCO3
7 (a) Give the names of the processes represented by the following equations: (1 mark)
(i) HCOOH(aq) H₂SO₄(1) CO(g) + H₂O(1)
(ii) Na₂CO₃.10H₂O(s) in air Na₂CO₃H₂O(s) + 9H₂O(1) (1 mark)
7(b)(ii) Concentrated sodium chloride solution, saturated with ammonia is passed into a carbonation tower in which carbon(IV) oxide is bubbled through. Reactions in the tower involve formation of ammonium hydrogen carbonate which then reacts with sodium chloride to form sodium hydrogen carbonate.
Write the equations for the formation of:
I. Ammonium hydrogen carbonate; (1 mark)
NH3(g) + CO2(g) + H2O(g) → NH4HCO3(aq) ✓1
II. Sodium hydrogen carbonate. ✓ (1 mark)
NH4HCO3(aq) + NaCl(aq) → NH4Cl(aq) + NaHCO3(s) ✓1
7 (b) (iii) Describe how the:
I. sodium hydrogen carbonate is separated; (1 mark)
By filtration ✓1 where sodium hydrogen carbonate is obtained as a residue and ammonium chloride solution as a filtrate ✓1
II. Sodium hydrogen carbonate is converted to sodium carbonate. (1 mark)
By thermal decomposition of NaHCO3 ✓1 or
2NaHCO3(s) --heat→ Na2CO3(s) + CO2(g) + H2O(l)
7 (b) (iv) One of the uses of sodium carbonate is in the removal of water hardness.
I. Explain how sodium carbonate removes water hardness. (1 mark)
Addition of sodium carbonate to hard water precipitates out Ca2+ or Mg2+ ions in form of their insoluble carbonates. ✓1 or
Through double decomposition to remove Ca2+ or Mg2+ from hard water.
II. State one other industrial use of sodium carbonate. (1 mark)
Manufacture of detergents, soaps, glass, paper ✓1
Manufacture of sodium silicate, borax, sodium phosphate